IBS Electronics, Inc.
*******************************
***
Quality Policy Manual
ISO9001:2000
Ver 6.0
Controlled Copy
IBS Electronics, Inc.
3506-D W. Lake Center Dr.
Santa Ana, Ca 92704 U.S.A.
714.751.6633
800.527.2888
714.751.8159 FAX
.
E-mail: ibs@ibselectronics.com
Internet: http://www.ibselectronics. com
quality_policy_manual_v6-html.html
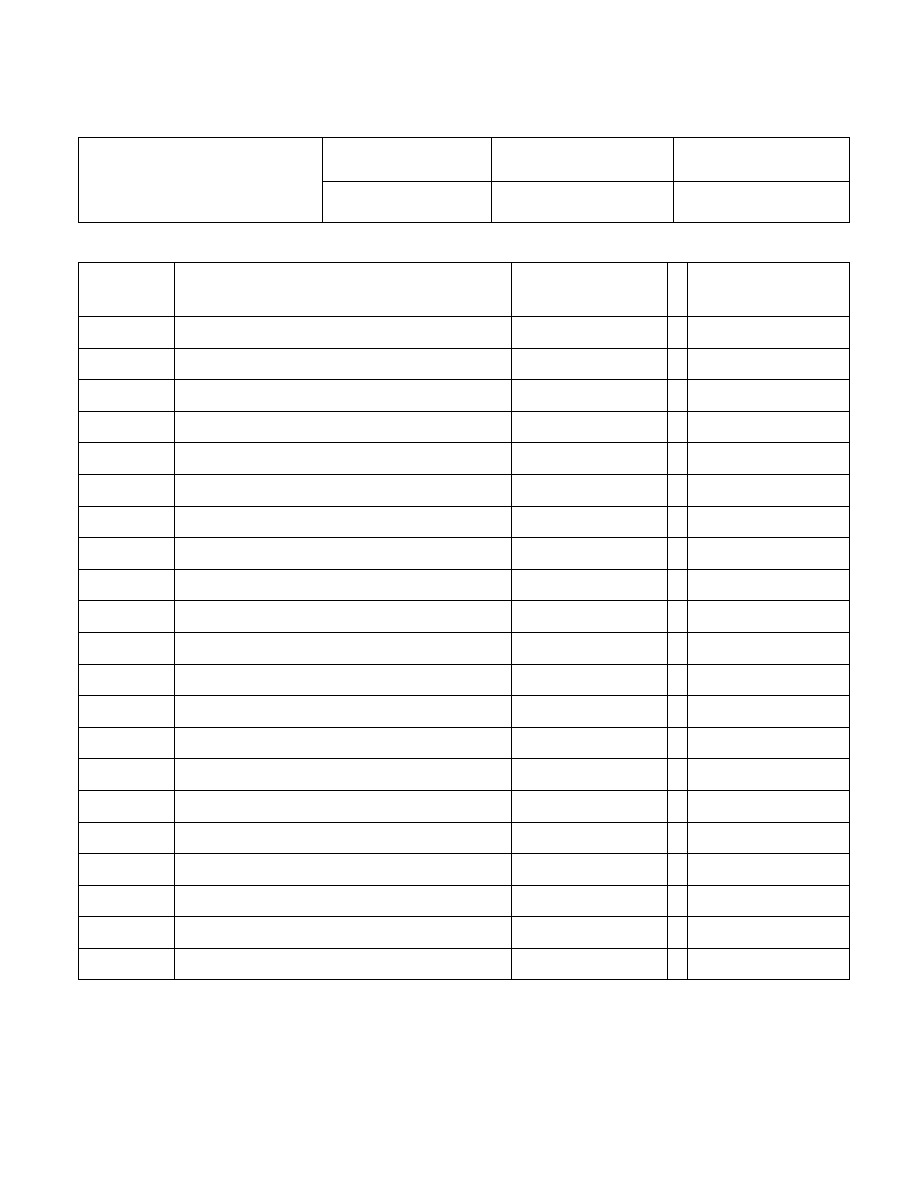
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 3
Title:
Table of Contents
Effective date:
11/22/06
Revision number:
6.0
Section :
A
Section
Number
Title ISO
9001:2000
Reference
Revision Date
A
Table of Content and Cross-reference
B
Quality Management System
1.0, 4.1, 5.3, and 5.4.1
C Planning
5.4.2
D Management
Responsibility
5.1, 5.2, and 5.5.3
E Organizational
Responsibilities
5.5.1 and 5.5.2
F
Management Review
5.6 all
G
Documentation Requirements
4.2 all
H
Resource Management
6 all
I Product
Realization
7.1
J
Customer Related Processes
7.2 all
K
Design and Development
7.3 all
L Purchasing
7.4
all
M
Production and Service Provision
7.5 all
N
Control of Monitoring and Measuring Devices
7.6
O
Measurement, Analysis and Improvement
8.1
P
Monitoring and Measurement
8.2 all
Q
Control of Nonconforming Product
8.3
R
Analysis of Data
8.4
S Improvement
8.5
all
T
Glossary (Terms & Definitions)
U
Record of Revision
6.0
quality_policy_manual_v6-html.html
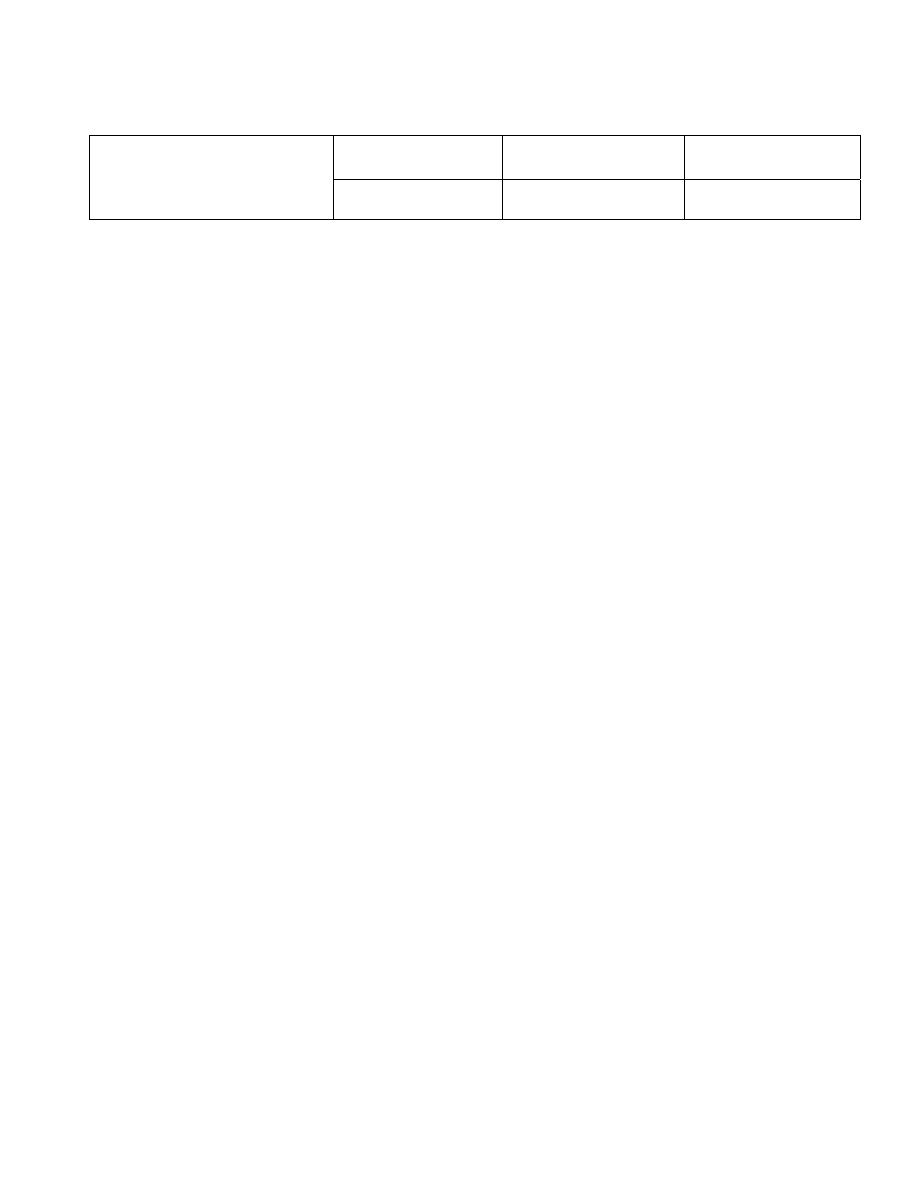
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 3
Title:
Table of Contents
Ref Documents
Effective date:
11/22/06
Revision number:
6.0
Section :
A
Reference Documents:
ISO 10005
Quality management - Guidelines for quality plans
ISO 9001-2000
Quality management systems-Requirements
ISO 9000-2000
Quality management systems-Fundamentals and vocabulary
ISO 9004-2000
Quality Management systems-Guidelines for performance
improvements
ISO 10011
Guidelines for Auditing Quality Systems
ISO10013
Guidelines for developing quality manuals
ISO9004-2000
Quality management systems-Guidelines for performance
improvements
quality_policy_manual_v6-html.html
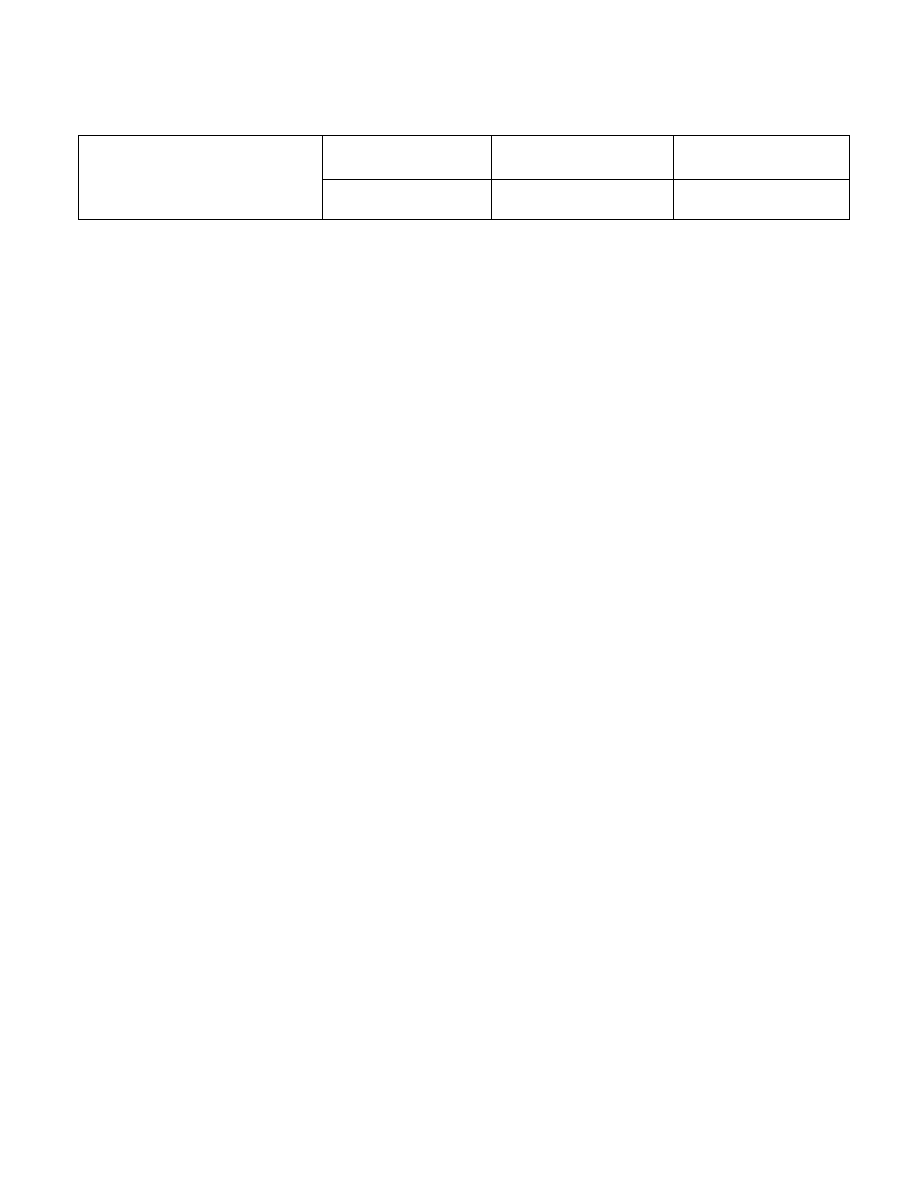
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 3
Title:
Table of Contents
IBS Information
Effective date:
11/22/06
Revision number:
6.0
Section :
A
IBS Electronics, Inc. Information
IBS Electronics, Inc. established in 1980, is a growing electronic components
distributor serving OEM customers, contract manufacturing and brokers that inquire
tailored inventory ,
industrial,
commercial,
mil-specs and aerospace components,
fast order processing, competitive pricing,
same day shipping,
technical and cross reference support,
just-in-time program,
value added services,
and total support.
This quality policy manual is issued and controlled by IBS management. The policies
defined in this manual is designed to meet the ISO 9001-2000 requirements.
Signed at Santa Ana, California
Signature in File: _________In File____________________ Date:11/22/06
GM,
CEO,
President
Signature in File: _________In File____________________ Date:11/22/06
Operation
Manager
Signature in File: _________In File____________________ Date:11/22/06
Quality
Manager,
Quality
Rep.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0
PURPOSE
1.1 This section is to specify
IBS
's requirements for a Quality Management
System (QMS) in order to demonstrate its ability to consistently provide
product
that meets
customer
requirements, and aims to enhance customer
satisfaction.
•
IBS excludes element 7.3, Design and development,
because IBS does not perform the activities.
•
IBS excludes element 7.5.4, customer supplied properly,
because IBS does not perform the activities.
•
IBS
excludes
element 7.5.2, Validation of processes for
production and service provision, because IBS does not
perform the activities.
2.0
REFERENCE DOCUMENTS
2.1
ISO 9001:2000 Clause 1.0, 4.1, 5.3, 5.4.1
2.2
Quality System Procedure – 1.2 Quality System Procedure
2.3
Quality System Procedure – 2.7 Standard Procedure Format
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1 Scope
4.1.1 IBS Electronics operates a quality system to meet the requirements of
ISO 9001-2000. The quality system is described in this manual and all
employees are to follow the elements of its content.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
4.1.2 The mission statement of IBS Electronics is:
TO BE THE RECOGNIZED COMPONENT
DISTRIBUTOR SPECIALIST IN DOMESTIC AND
OVERSEAS TO ACHIEVE CUSTOMER
SATISFACTION
The purpose of this manual is to provide:
1. A coordinated and systematic approach to quality throughout IBS
Electronics.
2. Guidance for the planning of all activities related to quality.
3. Guidance for IBS employees in defining their roles in quality.
4. An overview of IBS quality system for customers and suppliers.
4.2
Quality Management System - General
(4.1)
4.2.1 IBS has established, documented and implemented a Quality
Management System and continually improves its effectiveness in accordance
with the requirements of the ISO 9001-2000 Standards. IBS has:
a) identified the processes needed for its QMS and their application
throughout the company.
b) determined the sequences and interaction of these processes.
c) determined the criteria and methods needed to ensure that both the
operation and control of these processes are effective.
d) ensured the availability of resources per section H, and information
necessary to support the operation and monitoring of these processes.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
e) established criteria and means to effectively operate, monitor,
measure, analyze and control the processes including improvement of
quality management system effectiveness and improvement of these
processes per section R and S.
f) implemented actions necessary to achieve planned results and
continued improvements of these processes.
4.2.2 IBS does identify, outsource and ensure drop shipment process affecting
product with these requirements, Should any other process be outsourced IBS
will identify them and ensure control over such processes.
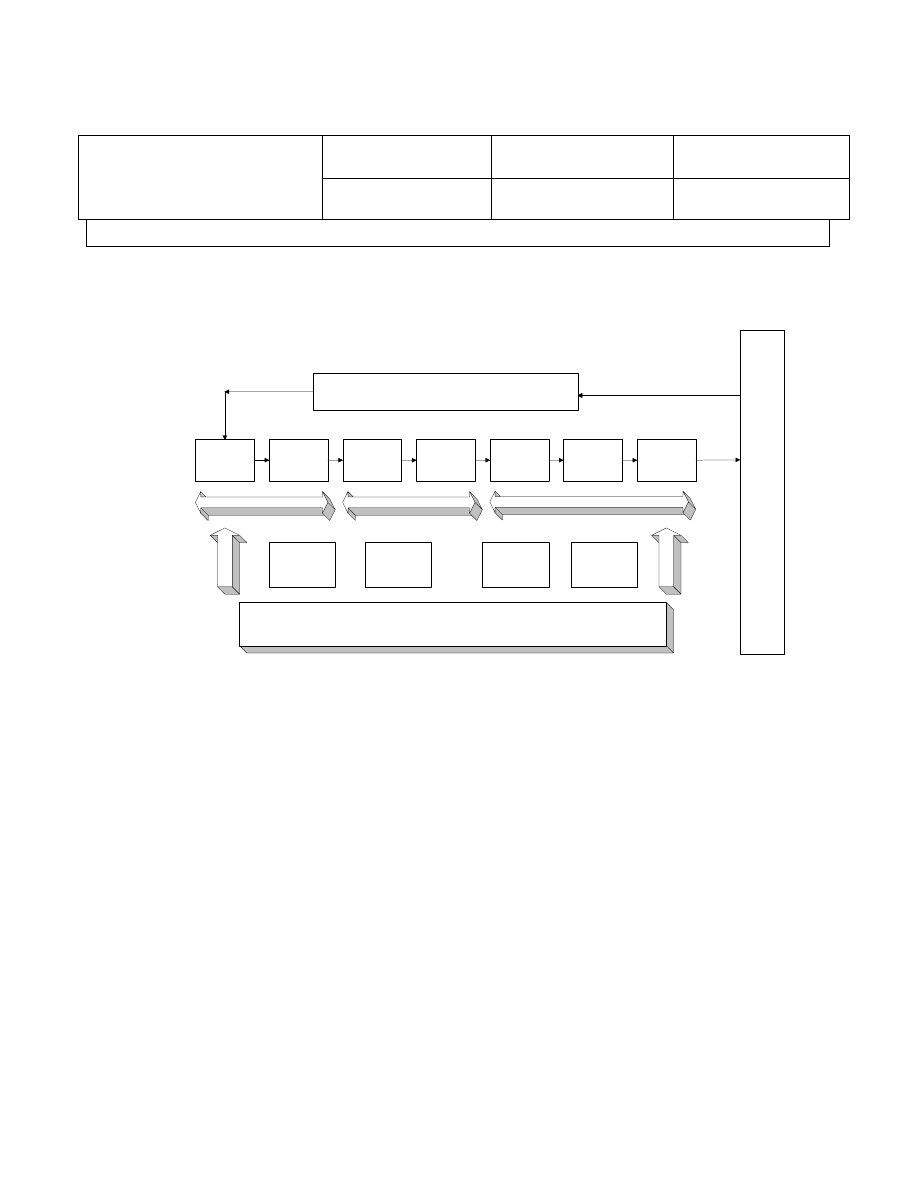
4.2.3 The following Key Processes have been identified and documented:
a) Sourcing and Quoting
b) Sales and Purchase Order
c) Order Processing
d) Sales and Purchasing Management
e) Quality Assurance Management
f) Human Resource Management
g) Financial Management
The interrelationship sequences and interactions are described on enclosed
diagram and detailed in section H. Supporting documentation and records are
developed as required.
4.2.4 The diagram on next page shows interaction of processes for IBS
Electronics:
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
4 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
Customer
Request
Process
Source &
Quote Material
Process
Review Order
Process SO &
PO
Process
Receive
Material
Process
Inspect &
Package
Material
Process Ship
Order
Customer Inquiry or Response
Customer
Sales Associate manages Customer Account
Sales and
Purchasing
Management
Quality
Assurance
Management
Human
Resources
Management
Financial
Management
Sourcing & Quoting
Management and Support Processes
Sales and Purchase Order
Order Processing
Process Map Diagram
Operation Management
4.3
Quality Policy (5.3)
4.3.1 The following quality policy, established by the management of IBS, has
been presented to all employees to be part of their orientation for Quality
System.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
5 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
IBS Electronics’ quality policy is
committed to provide quality parts and
services that satisfy our customers’
expectations on time, every time.
4.3.2 This policy is accomplished through educating all employees who are
parts of one IBS team on:
1.0 Total Customer Satisfaction
2.0 Commitment to Continual Improvement of the
effectiveness of quality management system. (5.3b)
3.0 All Employees Participation
4.4.
Quality Policy Implementation
4.4.1 This quality manual documents IBS quality system. It defines the
organizational structure, quality responsibilities and practices used to
implement quality related activities.
4.4.2 The quality system interacts with all employees from sales through final
inspection and customer support. It encourages continual improvement of
processes. Customer 's requirements are an integral part of quality system.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
6 of 6
Title:
Quality Management
System
Effective date:
11/22/06
Revision number:
6.0
Section :
B
Applicability: This section is applicable to all
IBS Electronics
operations.
4.5
Quality Objectives (5.4.1)
4.5.1 Objective evidences are documented records and all actions performed
daily by IBS employees’s work-related to the IBS quality system. These are
satisfactory proof of the established quality system. The overall quality
objectives are as follows:
•
continual
improvement of quality system
•
customer
satisfaction
•
maintain ISO 9001:2000 program
•
conforming to customer’s requirements
•
record of on time delivery
4.5.2 Measurement objectives
•
measurement of effectiveness of improvements by gathering and
analyzing internal audit report and audit data
•
measurement of customer satisfaction by gathering and analyzing
customer survey response data.
•
measurement of on time delivery by gathering and analyzing sales
performance and delivery history data.
•
measurement of meeting customer’s requirement by gathering and
analyzing customer returns and customer repeat business data
5.0 RESPONSIBILITIES
5.1
It is the responsibility of top management to ensure that the quality
policy is implemented and understood by all of IBS Electronics’ employees..
6.0 RECORDS
6.1 Records of Quality Objectives are a part of Management Review
process.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 1
Title:
Planning
Effective date:
11/22/06
Revision number:
6.0
Section :
C
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the planning of IBS Electronics, Inc.’ s QMS and
quality objectives.
2.0 REFERENCE
DOCUMENTS
2.4
ISO 9001:2000 Clause 5.4.2
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0
QUALITY MANAGEMENT SYSTEM REQUIREMENTS
4.1
Quality Management System Planning (5.4.2)
4.1.1 The sales, finance and operation managements define the requirements
and the quality representative documents the procedures.
4.2 Plan
Summary
4.2.1 IBS Electronics operates a quality system to meet the requirements of
ISO 9001-2000. The quality system is described in this manual and all
employees are to follow the elements of its content. Planning is accomplished
during management review and other management meetings. Four primary
groups shapes IBS quality system: Sales, Operation, Finance and Quality as
described in section E.
5.0 RESPONSIBILITIES
5.1 The Quality policy, established by management of IBS Electronics, has
been presented to all employees to be part of their orientation for quality
system.
6.0 RECORDS
6.1 The Records for QMS planning are maintained through documented
evidence.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Management
Responsibility
Effective date:
11/22/06
Revision number:
6.0
Section :
D
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes top management’s responsibilities with regard to
the continual improvement of IBS’ Quality Management System and the
enhancement of customer satisfaction.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clauses 5.1, 5.2 and 5.5.3
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Management Commitment (5.1)
4.1.1 IBS management provides evidences of commitment to the QMS, and to
continual improvement of the effectiveness of the QMS, by
•
communicating to all employees the importance of meeting customer
requirements.
•
establishing the quality policy, and ensuring that this policy is
understood by all employees.
•
ensuring that the quality objectives are established.
•
conducting management reviews
•
ensuring the availability of resources
4.2
Customer Focus (5.2)
4.2.1 IBS management ensures that customer requirements are met.
Customer requirements are identified in section J, para 4.1, 4.2 and 4.3 and
customer satisfaction is covered in section P, para 4.1.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Management
Responsibility
Effective date:
11/22/06
Revision number:
6.0
Section :
D
Applicability: This section is applicable to all
IBS Electronics
operations.
4.3
Internal Communication (5.5.3)
4.3.1 IBS management provides evidence of commitment to the QMS, and to
continual improvement of the effectiveness of the QMS. IBS management
ensures that appropriate communication processes are established and that
communication takes place regarding the effectiveness of the QMS
4.3.2 This task is accomplished by IBS newsletter published monthly or
quarterly, scheduled top management meeting and by e-mails and
correspondences from management to employees. Daily morning
sales/purchasing meeting are also part of internal communication.
5.0 RESPONSIBILITIES
5.1 IBS management is responsible to ensure customer focus and internal
communication is implemented.
6.0 RECORDS
6.1 The quality records are documented within this QMS.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 3
Title:
Organization
Responsibility
Effective date:
11/22/06
Revision number:
6.0
Section :
E
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section defines the responsibilities and authorities of IBS personnel
for implementing and maintaining the Quality Management System (QMS).
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clauses 5.5.1 and 5.5.2
2.2
Quality System Procedure – 1.3 Organization Responsibility
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Responsibility and Authority (5.5.1)
4.1.1 The responsibility, authority and interrelation of employees who
manage, perform and verify quality is shown on the IBS chart in this section
(see also IBS organization chart).
General Manager
Sales
System Operation
Finance
Quality
4.1.2 For purposes of this section IBS is composed of primary special groups:
Sales, Finance, Operation and Quality. The responsibilities of all areas as
follows:
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 3
Title:
Organization
Responsibility
Effective date:
11/22/06
Revision number:
6.0
Section :
E
Applicability: This section is applicable to all
IBS Electronics
operations.
•
sales:: domestic and international sales, post sales customers
maintenance.
•
finance (accounting): general book keeping , ledgers and internal audits
•
system operation:: purchasing, receiving, storage and shipping of the
parts.
•
quality: quality records, audits, corrective actions and inspection
4.1.3 IBS leadership has committed to providing adequate resources. and
personnel sufficient to obtain the quality program goals. Audit group is the
vehicle for verifying the success of the quality system. Audit group consists of
quality rep and internal auditor.
4.2
Management Representative (5.5.2)
4.2.1 The Quality Representative (Management Representative) is responsible
to the General Manager for leading, monitoring and auditing all quality related
activities and for reporting on all quality matters.
4.3
Quality Assurance Manager
4.3.1 The Quality Assurance Manager is a management representative for ISO
9001-2000. Quality Management System. The responsibilities are described
in paragraph 4.2 and 5.0.
5.0 RESPONSIBILITIES
5.1 The President/General Manager has the overall responsibility for the
definition of, and adherence to, the quality policy and through the Quality
Representative, for the authorization and implementation of the quality system.
5.2 The quality of IBS parts and services depends on each group effectively
performing tasks that contributes to, or affects, quality.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 3
Title:
Organization
Responsibility
Effective date:
11/22/06
Revision number:
6.0
Section :
E
Applicability: This section is applicable to all
IBS Electronics
operations.
5.3 The manager of each group is responsible for the work performed by each
member and for ensuring that the members of each group are appropriately
qualified for their assigned level of authority and responsibility.
5.3 The Quality Representative has the authority and responsibility for
ensuring that the requirements of IBS's Quality Policy are implemented and
maintained. While carrying out this responsibility, the Quality Representative
will report to the IBS President/General Manager.
6.0 RECORDS
6.1 The job classification, relating to the skills, expertise and knowledge of
people shown on the chart are maintained in employee benefits. The
descriptions may be amended by general manager directive or local, state and
federal laws.
For purpose of this section below documents are provided:
Organizational Chart
Job Descriptions
6.2 Organizational chart and Job description are added to this QMS and
they can be revised at any time to reflect present changes.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Management
Review
Effective date:
11/22/06
Revision number:
6.0
Section :
F
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0
PURPOSE
1.1 This section establishes the requirements for top management’s review of
the Quality Management System to ensure its continuing suitability,
adequately, and effectiveness.
2.0
REFERENCE DOCUMENTS
2.1
ISO 9001:2000 Clause 5.6
2.2
Quality System Procedure – 1.4 Management Quality Review
3.0
DEFINITIONS
3.1
See Section 20, Glossary, for definitions
4.0
QMS REQUIREMENTS
4.1
Management Review – General (5.6.1)
4.1.1 The review will include consideration of the suitability and effectiveness
of the quality system and the effectiveness of corrective actions taken since
the last management review. Top management review IBS’ QMS on semi-
annual basis.
4.2
Review Inputs (5.6.2)
4.2.1 As a minimum, the following subjects will be considered during the formal
management review of the quality system:
Internal
Audits
status of preventive and corrective actions
follow up actions from previous management reviews
customer
feedback/complaints
changes that could affect the quality management system
management quality objectives/recommendations for improvement
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Management
Review
Effective date:
11/22/06
Revision number:
6.0
Section :
F
Applicability: This section is applicable to all
IBS Electronics
operations.
4.3
Review Output (5.6.3)
4.3.1 Any conclusion or action item will be recorded and assignee will be
determined for that action item.
management objectives on quality system
.
training plan and recommendations for improvement and preventive, corrective
actions and preventive plan.
Improvement of the effectiveness of the quality management system.
5.0 RESPONSIBILITIES
5.1 The quality system will be reviewed by the President/General Manager and
Quality Rep. to improve the quality system.
6.0
RECORDS
6.1 The quality Representative will maintain records of the management
review and internal audits for specified period as described in section G.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 5
Title:
Documentation
Requirements
Effective date:
11/22/06
Revision number:
6.0
Section :
G
Applicability: This section is applicable to all IBS Electronics operations.
1.0 PURPOSE
1.1 This section establishes the requirements for documentation of the Quality
Management System (QMS). The system provides for the uniform preparation,
revision, distribution, retrieval, and storage of documents and records.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Paragraphs 4.2.1, 4.2.2, 4.2.3 and 4.2.4
2.2
Quality System Procedure – 5.8 Document control
2.3
Quality System Procedure – 16.5 Quality records
3.0
DEFINITIONS
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Documentation Requirements - General (4.2.1)
4.1.1 IBS QMS documentation includes:
b) documented statements of a quality policy and quality objectives,
c) this Quality Manual
d) the documented procedures referenced within each section of
this Manual where required by the ISO9001:2000 Standards,
e) documents needed by the IBS organization to ensure the
effective planning, operation, and control of its processes, and
f) records required by the ISO 9001:2000 Standard.
4.2
Quality Manual (4.2.2)
4.2.1 The quality manual
4.2.1.1 This quality manual documents IBS quality system. It defines the
organizational structure, quality responsibilities and practices used to
implement quality related activities.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 5
Title:
Documentation
Requirements
Effective date:
11/22/06
Revision number:
6.0
Section :
G
Applicability: This section is applicable to all IBS Electronics operations.
4.2.1.2 The quality system interacts with all employees from sales through final
inspection and customer support. It encourages continual improvement of
processes. Customer requirements are an integral part of the quality system.
4.2.2 Policy Update
4.2.2.1 Quality policy manual and procedures are updated when necessary
under the authority of the quality representative and copies of changed sheets
will be distributed to all personnel affected and the original in the manual and
database will be replaced.
4.2.3 Manual and Procedures Changes
4.2.3.1 Any IBS employee may submit proposed quality manual and procedure
changes to the quality representative.
4.2.3.2 All controlled document changes and modification must be approved
by the President/General Manager or Quality Representative.
4.2.3.3 Any quality policy changes, will affect this version of the quality policy
manual. The version letter changes to next character and description of the
change(s) will be recorded.
4.2.3.4 When revised documents are circulated, a change brief is included on
first section of page one which outlines where to look for changes.
4.2.3.5 The alternative method of latest revision of revised documents is
circulated through the system network. The revised documents are in Intranet.
All employees have access to review all procedures and company policy
whenever needed.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 5
Title:
Documentation
Requirements
Effective date:
11/22/06
Revision number:
6.0
Section :
G
Applicability: This section is applicable to all IBS Electronics operations.
4.2.3.6 IBS established and maintains this Quality Manual that includes:
The scope of the QMS, as it applies to products and services. The
justifications for exclusions claimed under ISO 90001:2000 Standard
are detailed below.
•
IBS does not hold Design and development for exclusion of
element 7.3.
•
IBS does not hold any customer supplied properly for
exclusion of element 7.5.4.
4.3
Control of Documents (4.2.3)
4.3.1 The document control procedures define the requirements for developing
methods for controlling the generation, modification, approval and distribution
of documents. The quality rep has the responsibility for the document control
processes.
4.3.2 IBS Quality Manual is a controlled document under the responsibility of
quality representative. At present time one master copy of quality manual is
available.
4.3.3 The system network is also available for employees to view the latest
revisions of the Quality Manual and procedures. The files are located in
Intranet under ISO link. A Master List has been established to identify the
current revision of documents in order to control the issuance and revision
status of the documents.
4.4
Control of Records (4.2.4)
4.4.1 Procedures are established and maintained for identification, collection,
indexing, filing, storage, maintenance, and disposition of quality records.
Quality records collected provide traceability and allow analysis of trends and
conformance to requirements.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
4 of 5
Title:
Documentation
Requirements
Effective date:
11/22/06
Revision number:
6.0
Section :
G
Applicability: This section is applicable to all IBS Electronics operations.
4.4.2 Quality records are developed to support requirements of traceability,
conformance to requirements and continual improvement processes.
4.5 Documentation
Structure
4.5.1
The Operation Procedures
4.5.1.1 The quality system documentation structure is based on a quality policy
and procedures established.
4.5.2 The Document Control System assures that all controlled documents and
data affecting purchasing and quality are current and approved for release per
the applicable procedures. Controlled document include, but are not limited to:
a. Operation Procedures
b. Quality Manual
5.0 RESPONSIBILITIES
5.1 The quality representative maintains the documented quality system as
described in this manual. The manual is used as a means of ensuring that
parts and services conform to the requirements of ISO 9000-2000.
5.2 The document control process is managed by quality rep. and quality
policy and manual and documents are kept under one master copy.
Sales, Inventory, Accounting and Quality are responsible for ensuring
complete and accurate records are kept to support customer access to quality
records. The responsibilities for record maintenance and retention include:
-Verification that records are legible and identifiable to demonstrate
achievement of required quality system.
-Records are stored in such a manner that they are easily retrievable
and protected from deterioration.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
5 of 5
Title:
Documentation
Requirements
Effective date:
11/22/06
Revision number:
6.0
Section :
G
Applicability: This section is applicable to all IBS Electronics operations.
6.0 RECORDS
6.1 IBS requires that a master list of the latest revisions of all controlled
documents be maintained by Quality Rep.
6.2 The management of records is the responsibility of each of the employee.
All quality records are maintained either in filling cabinets for paper or in a data
base for electronically collected records.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 4
Title:
Resource
Management
Effective date:
11/22/06
Revision number:
6.0
Section :
H
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the requirements for the management of the
resources that are essential to the implementation and continual improvement
of the QMS.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause
Paragraphs 6.1, 6.2 and 6.3
2.2
Quality System Procedure – 18.5 Quality Training and Education
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Provision of Resources - General (6.1)
4.1.1 IBS leadership has committed to providing adequate resources. and
personnel sufficient to attain the quality program goals. Audit process is the
vehicle for verifying the success of the quality system. When resources
requirements change, management ensures that adequate resources are
allocated.
4.2
Human Resources – General (6.2.1)
4.2.1 All employees are verified to be competent in their specific job
assignments on the basis of appropriate education, training, and experience.
4.2.2 The job classification, relating to the skills, expertise and knowledge of
people shown on the chart are maintained in employee folders. The
descriptions may be amended by President/General manager directive or
local, state and federal laws.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 4
Title:
Resource
Management
Effective date:
11/22/06
Revision number:
6.0
Section :
H
Applicability: This section is applicable to all
IBS Electronics
operations.
4.3
Competence, Awareness and Training (6.2.2)
4.3.1 A training program is created to specify the requirements, type and
content of training for new and existing employees.
4.3.2 A training program is implemented and applies to all employees
performing specific task affecting quality. All employees are qualified on the
basis of education, training, or experience as needed.
4.3.3 As part of orientation process, all new employees receive employee
handbook. Employee handbook contains information about the IBS
employment policies and practices.
4.3.4 As part of training program, IBS evaluates the effectiveness of actions
taken and ensures that personnel are aware of the relevance and importance
of their activities and how they contribute to the achievement of the quality
objectives.
4.3.5 Employee Feedback
4.3.5.1 IBS is interested in employee’s constructive ideas and suggestions for
improving operation and continual improvement. Process is established to
obtain employee‘s response or supervisor’s response on completed subject
training.
4.4 Infrastructure
(6.3)
4.4.1 IBS determines, allocates, provides and maintains the needed
infrastructure to comply with requirements including:
•
buildings, workplace and related utilities
•
process equipment (both hardware and software)
•
any supporting services as applicable
4.4.2 IBS utilizes controls, where appropriate, that consists of procedures,
process audits to maintain product quality. Buildings, workplace and
associated utilities Process equipment such as computers and all necessary
softwares and databases Supporting services such as transportation/delivery
companies and communication devices (telephone, fax and e-mails)
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 4
Title:
Resource
Management
Effective date:
11/22/06
Revision number:
6.0
Section :
H
Applicability: This section is applicable to all
IBS Electronics
operations.
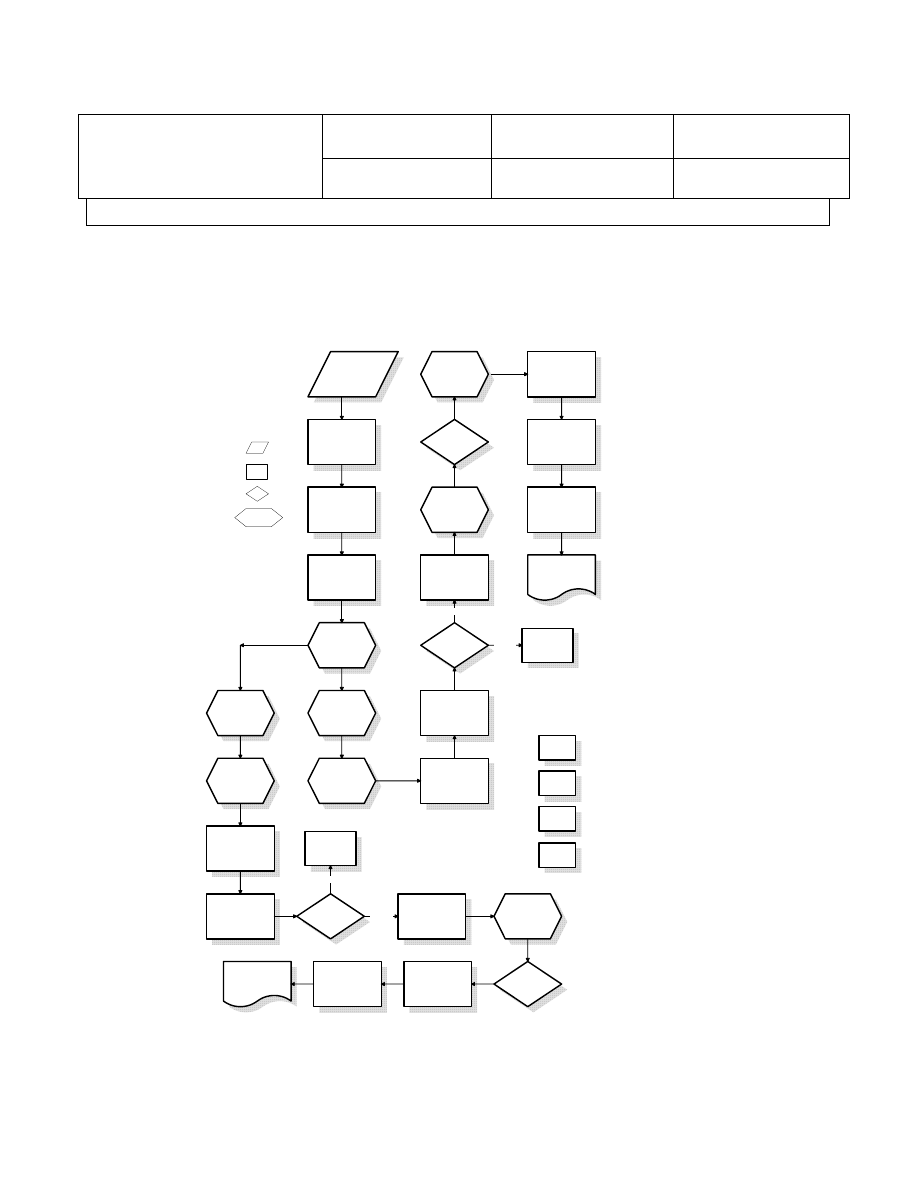
4.4.3 Master flow chart
4.4.3.1 Master operation flow chart is created. It defines the operational steps
for completing the customer requirements on consistent basis.
Receive
requirements
from
customer
Source the
requirements for
customer
Quote the
requirements for
customer
Receive customer
purchase order for
the requirements
Setup part
number per
requirements
Create sales
order from
customer
purchase
order
Order the material for
customer
Create IBS
Purchase
Order
Receive material from
Supplier
Inspect material per
purchase order
Search for sales
order
Issue the pick
list
Inspect material per
sales order/pick list
Pack material
Return to
supplier
Use customer's
selected carrier
Issue the
invoice
File records
Ship material
Accepted
Rejected
Master Operation Flow
Chart
Issue Date: 11/14/95
Revised: 06/15/05
Data
Process
Decision
Preparation
IBS Electronics, Inc.
Sales
Group
Operation
Group
Quality
Group
Support Groups
HONG KONG
DROP SHIPMENT,
YES
HONG KONG DROP
SHIPMENT,
NO
Create sales
order from
customer
purchase order
for hong kong
location
Create IBS
Purchase
Order for
hong kong
location
Order the material for
customer
Receive material from
Supplier at hong
kong location
Inspect material per
purchase order
Search for sales
order in data base
Inspect material per
sales order/invoice
Issue the
invoice
Pack material
Use Customer's
selected Carrier, ship
material
File records
Return to
supplier
Rejected
Accepted
Financial
Group
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
4 of 4
Title:
Resource
Management
Effective date:
11/22/06
Revision number:
6.0
Section :
H
Applicability: This section is applicable to all
IBS Electronics
operations.
4.5
Work Environment (6.4)
4.5.1 IBS determines and manages the work environment on human factors
such as work method, safety rules/guidance, if any; and physical factors
such as, cleanliness and airflow. IBS determines if it is necessary to control
work areas for human and physical factors. IBS management ensures that
different activities work areas are not mixed up.
5.0 RESPONSIBILITIES
5.1 All managers are responsible to identify training needs and to provide the
required training for all personnel whose jobs affect quality.
5.2 New employee education and experience are verified by hiring Manager
when that education or experience is to be used in the place of on the job
training.
5.3 All managers and supervisors are responsible for employees feedback,
suggestions and ideas on training and quality systems. The results will be
collected and report will be created.
6.0 RECORDS
6.1 Quality Rep. up to the date of termination maintains the training records of
employees. IBS document a formal, annual training plan to address the
training needs of personnel. The plan will be revised or updated for change
every six months.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Product
Realization
Effective date:
11/22/06
Revision number:
6.0
Section :
I
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the requirements for planning and developing the
processes needed for product realization.
2.0
REFERENCE DOCUMENTS
2.1
ISO 9001:2000 Clause 7.1
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Planning of Product Realization (7.1)
4.1.1 The quality representative maintains the documented quality system as
described in this manual. The manual is used as a means of ensuring that
parts and services conform to the requirements of ISO 9001-2000.
4.1.2 The quality system interacts with all employees from sales through final
inspection and customer support. It encourages continual improvement of
processes. Customer requirements, are an integral part of quality system.
4.1.3 The quality system defines the organizational structure, quality
responsibilities and practices used to implement quality related activities which
is based on international procurement, warehousing, distribution and sale of
electronic components, electromechanical equipment, computer peripherals
and electronic industry chemical and added value services.
4.1.4 IBS plans and develops the processes needed for product realization.
Planning of product realization is consistent with the requirements of the other
processes as described in flow chart in section H.
5.0 RESPONSIBILITIES
5.1 The sales, accounting and operation define the requirements and quality
representative document the procedures.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Product
Realization
Effective date:
11/22/06
Revision number:
6.0
Section :
I
Applicability: This section is applicable to all
IBS Electronics
operations.
6.0 RECORDS
6.1 The quality system documentation structure is based on a quality policy
and procedures established. The planning evidences may be in various forms
including orders, checklists, and other documents.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Customer – Related
Processes
Effective date:
11/22/06
Revision number:
6.0
Section :
J
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes determining, reviewing, and communicating
product requirements for customer-related processes. And to ensure the IBS
has the capability to meet all customer-specified requirements
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 7.2
2.2
Quality System Procedure – 3.4 Customer Order Review and Entry
2.3
Quality System Procedure – 3.5 Customer Data Entry and Credit Approval
2.4
Quality System Procedure – 3.6 Fax Distribution
2.5
Quality System Procedure – 3.7 Order and Shipment Cancellation
2.6
Quality System Procedure – 3.8 Parts Sourcing and Quotation
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.0.1 The purpose of contract review is to clearly communicate customer
requirements to IBS through quotation and sales order processing system to
ensure successful delivery of parts and services that meet the customer's
needs.
4.1
Determination of Requirements Related to the Product (7.2.1)
4.1.1 The sales group manager is authorized to implement procedures for
sourcing, quotation and customer order review to ensure that IBS has clear
understanding of customer purchase orders.
4.1.2 Where the customer provides no documented statement of requirement,
the customer requirements and clarification are confirmed by IBS before
acceptance. (Ref.: Section, D, Para 4.2, Customer Focus)
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Customer – Related
Processes
Effective date:
11/22/06
Revision number:
6.0
Section :
J
Applicability: This section is applicable to all
IBS Electronics
operations.
4.2
Review of Requirements Related to the Product (7.2.2)
4.2.1 The review will include the verification that the customer needs are
clearly defined and documented and can be met within the specified time
frame. By entering product order in IBS system database, or via fax/e-mail,
IBS confirms meeting the defined requirements. Sales associate or sales
manager or general manager review and sign sales orders as required.
4.2.2 Contracts are reviewed, as a minimum. Quantities and ship-dates by the
sales group before acceptance, including the requirements for delivery activity.
The post delivery activity is limited to support of any quality issue
4.3
Customer Communication (7.2.3)
4.3.1 IBS management and sales group are communicating with customers on
daily basis. Any Changes to orders are subject to the same review processes
and guidelines as the original contract or order.
4.3.2 IBS uses technology such as network system (e-mails, faxes) and
telephone communication system (phone directory) for incoming customer
inquiries, customer feedback, or/and customer complaints for routing to
appropriate sales associate or quality representative.
.0 RESPONSIBILITIES
5.1 Sales associates) are responsible for the review of quotation and execution
of all sales orders generated by sales group activities.
6.0 RECORDS
6.1 The database file is maintained by sales for all sales orders. Records of
documentation and review exist as electronics file in computer database.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 1
Title:
Design and
Development
Effective date:
11/22/06
Revision number:
6.0
Section :
K
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
IBS Electronics, does not provide any design activities at present time
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 7.3
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Design and Development Planning (7.3.1)
4.2
Design and Development Inputs (7.3.2)
4.3
Design and Development Outputs (7.3.3)
4.4
Design and Development Review (7.3.4)
4.5
Design and Development Verification (7.3.5)
4.6
Design and Development Validation (7.3.6)
4.7
Control of Design and Development Changes (7.3.7)
5.0 RESPONSIBILITIES
6.0 RECORDS
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 3
Title:
Purchasing
Effective date:
11/22/06
Revision number:
6.0
Section :
L
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0
PURPOSE
1.1 This section establishes the requirements for verifying that purchased
product conforms to the specified purchasing agreements.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 7.4
2.2
Quality System Procedure – 6.5 Purchasing Order Documents
2.3
Quality System Procedure – 6.6 supplier Parts Assurance Requirements
2.4
Quality System Procedure – 6.7 Supplier Approval List (SAL)
2.5
Quality System Procedure – 6.8 Supplier Performance Measurements
System
2.6
Quality System Procedure – 6.10 Supplier Quality Profile Questionaire 6.9
3.0 DEFINITIONS
3.1
See Section 20, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Purchasing Process (7.4.1)
4.1.1 IBS's approach to materials purchasing and supplier selection criteria.
4.1.2 IBS purchases materials that conform to its requirements and will
contract with suppliers that adhere to its standards.
4.1.3 Supplier’s qualification will be verified by satisfactory past performance
(report from database), in the case of new suppliers, by first time buy, surveys,
test or other data, evaluation of part samples or other relevant information.
Suppliers are evaluated by Quality Rep. and authorized buyer.
4.1.4 Suppliers will be sent the (supplier parts assurance requirements, if
required) and supplier quality profile questionnaire for evaluation of their
quality systems and IBS internal records.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 3
Title:
Purchasing
Effective date:
11/22/06
Revision number:
6.0
Section :
L
Applicability: This section is applicable to all
IBS Electronics
operations.
4.1.5 A listing of complete approved sources, major sources and disqualified
sources are maintained in Intranet data base and are controlled by Quality
Rep.
4.2
Purchasing Information (7.4.2)
4.2.1
Purchasing information contains a clear and complete part number of
materials or services to be purchased. Each PO contains (as a minimum) part
numbers, quantities, descriptions and delivery dates as applicable. IBS
ensures the adequacy of any specified purchase requirements prior to
contacting the suppliers.
4.3
Verification of Purchased Product (7.4.3)
4.3.1 Inspection/verification is performed in accordance with documented
procedures. Measurement of the quality of received part is through visual
inspection.
4.3.2 Performing verification activities at the supplier’s premises are not
common for IBS organization. This requirement does not apply and if such a
situation ever arises, IBS will prepare a unique quality plan to address the
issue.
5.0 RESPONSIBILITIES
5.1 The Sales/Purchasing is responsible for the development and
implementation of IBS purchasing policy including approval process for review
and approval of purchasing documents before release to suppliers.
5.2 Shipping and receiving is responsible for the receipt, checking and
inventory of purchased materials per documented procedures.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 3
Title:
Purchasing
Effective date:
11/22/06
Revision number:
6.0
Section :
L
Applicability: This section is applicable to all
IBS Electronics
operations.
6.0 RECORDS
6.1 Records of reviewed purchasing documents are filed and maintained for
compliance to the requirements.
6.2 Records of verification of purchased product maintains as receiving
records (inspection records).
6.3 Purchased items in stock are identified by a part number and are stocked
by a location code in computer data base.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 4
Title:
Production and Service
Provision
Effective date:
11/22/06
Revision number:
6.0
Section :
M
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the requirements for
production and service
provision
related to the international procurement, warehousing, distribution
and sale of electronic components, electromechanical equipment, computer
peripherals, and electronic industry, chemicals, and added value services.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 7.5
2.2
Quality System Procedure – 6.9 Return of materials & issuance of debit
memo
2.3
Quality System Procedure – 8.5 Parts number Assignment and Parts Setup
2.4
Quality System Procedure – 8.6 Products Identification and Traceability
2.5
Quality System Procedure – 19.4 Receipts of RMA & issuance of credit
memo
2.6
Quality System Procedure – 15.4 Handling for Electro-Static Discharge
Sensitive (ESDS) items.
2.7
Quality System Procedure –15.5 Material Handling and Storage (General
Guidelines)
2.8
Quality System Procedure – 15.6 Shipping
2.9
Quality System Procedure –15.7 Packaging Instructions and Delivery
2.10
Quality System Procedure – 15.8 Limited Shelf Life Material (General
Guidelines)
2.11
Quality System Procedure – 15.9 Inventory Cycle
2.8
Quality System Procedure – 12.5 Stamp control
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 4
Title:
Production and Service
Provision
Effective date:
11/22/06
Revision number:
6.0
Section :
M
Applicability: This section is applicable to all
IBS Electronics
operations.
4.1
Control of Production and Service Provision (7.5.1)
4.1.1 The servicing is limited to customer return and replacement order.
4.1.2 Procedures are established for control of nonconforming parts
determined by customer. Completed records include, Return Material
Authorization Number, identification and quantity of materials returned, reason
for return and corrective action follow-up as required.
4.2
Validation of Processes for Production and Service Provision (7.5.2)
4.2.1 At IBS, Production and Service provision are not required IBS validates
any processed thru use of methods, procedures and quality records.
The inspection system includes the use of customer and part specification,
and/or support of sales group and/or processing of incoming and outgoing
products in the computer system. Sales group and quality Rep. will ensure all
materials meet IBS's standards by documented procedures. These controls
ensure parts received, stored via appropriate verification. Nonconforming parts
are segregated and discrepancies are resolved by Quality Rep. and Sales
Group.
4.3
Identification and Traceability (7.5.3)
4.3.1 Identification is maintained for all products received.
4.3.2 Product identification and traceability activities are controlled by the
appropriate procedures, which provide levels of identification and control to
prevent mixing conforming to non conforming product. Product status and
control exist from receiving through shipment to the customer.
4.4
Customer Property (7.5.4)
4.4.1 At IBS, Parts and materials are not supplied by the customer for
incorporation into any supplies or related activities. All materials are purchased
direct from supplier.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 4
Title:
Production and Service
Provision
Effective date:
11/22/06
Revision number:
6.0
Section :
M
Applicability: This section is applicable to all
IBS Electronics
operations.
4.5
Preservation of Product (7.5.5)
4.5.1 Materials, components are protected from damage during storage,
handling and shipping.
4.5.2 The care is exercised when handling materials, components or products
from receipt through shipment. All components are handled in accordance with
appropriate procedures (control of non conforming materials, ESD controls,
etc.)
4.5.3 Materials are stored under conditions that prevent their deterioration,
contamination or damage. Storage of raw materials is monitored to ensure
proper and safe use of stored materials.
4.5.4 Packaging requirements are specified to adequately protect products
during vendors shipments to IBS and during IBS shipments to customers.
Special packaging conditions and requirements are documented on the
applicable sales or purchase orders.
4.5.5 IBS strives to ensure that the product is delivered in a method that
ensures the product will not see any damage during normal handling. Packing
and shipping ensures that any customer specified shipping requirements are
met.
5.0 RESPONSIBILITIES
5.1 The Sales and Quality are responsible for customer returns and all follow-
up activities.
5.2 The Quality rep. is responsible for implementing the procedure to inspect
the materials.
5.3 Sales and Inventory Control share the responsibility of product
identification and traceability.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
4 of 4
Title:
Production and Service
Provision
Effective date:
11/22/06
Revision number:
6.0
Section :
M
Applicability: This section is applicable to all
IBS Electronics
operations.
5.4 The Quality Rep. is responsible for procedures to implement the
appropriate controls and instructions for handling, storage, packaging,
preservation and delivery.
5.5 The shipping personnel are responsible for delivery of products to the
customer and to ensure that the materials reach the customer in good
conditions.
6.0 RECORDS
6.1 Traceability of products shipped to each customer is maintained by daily
shipping log, invoice numbers, sales orders and customer purchase orders.
through Sales, Accounting and inventory control. The system database can
create required reports.
6.2 Receiving and shipping records (final inspection records) will be kept
attesting to the effective operation of this policy
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 1
Title:
Control of Monitoring and
Measuring Devices
Effective date:
11/22/06
Revision number:
6.0
Section :
N
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes requirements for control of monitoring and
measuring to be undertaken by IBS Electronics, Inc.
1.2 At present time IBS’ calibration program is limited to one counting scale.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 7.6
2.2
Quality System Procedure – 11.5 Calibration and Inspection of Test
Equipment
s (for Future use).
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0
QMS REQUIREMENTS (7.6)
4.1 IBS owned equipment, used in the testing as required, is calibrated as
described herein.
4.2 Outside supplier is contracted to calibrate and maintain test equipment that
is used to demonstrate parts compliance with quality requirements. The
inventory control equipment is calibrated in house with appropriate procedures.
5.0 RESPONSIBILITIES
5.1 The Quality representative is responsible for the calibration and
maintenance program. The Quality Rep. will ensure calibration requirements
are performed and equipment not calibrated is clearly identified.
6.0 RECORDS
6.1 Calibration certificates, calibration types with calibration and recalibration
dates of test equipment are maintained and kept by the Quality Rep.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Measurement, Analysis, and
Improvement
Effective date:
11/22/06
Revision number:
6.0
Section :
O
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the
requirements
for the measurement, analysis,
and improvement of the Quality Management System (QMS) processes.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Clause 8.1
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0
QMS REQUIREMENTS (8.1)
4.1 The monitoring, measurement, analysis and improvement of data are
defined by management of IBS.
4.2 The determination of specific analysis of data requirements depend on the
need for control as defined by management.
a)
customer satisfaction report,
b)
findings from internal quality system audits
c) nonconformance
records
d) supplier
performance
e)
statistical process analysis on sales performance
f)
results from management reviews
g)
records of customer complaints.
4.3 IBS uses cost of quality reports, scrap reports, nonconformance reports,
final inspection to insure product conformity to requirements
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Measurement, Analysis, and
Improvement
Effective date:
11/22/06
Revision number:
6.0
Section :
O
Applicability: This section is applicable to all
IBS Electronics
operations.
4.4 IBS ensures conformity to the QMS through internal and external audits,
management reviews, and analyzing nonconformance and customer feedback.
4.5 IBS uses quality reports, the results of internal audits, nonconformances,
corrective and preventive action reports, and customer feedback to continually
improve the effectiveness of the QMS.
4.6 IBS communicates the measurements and analysis for improvement to the
affected party for appropriate action.
5.0 RESPONSIBILITIES
5.1 The Quality Rep. is responsible for implementing the method of and
procedures that will measure the process performance as required.
5.2 Documented procedures are generated as required to establish part
conformance to the requirements of purchase order.
5.3 Sales, receiving and quality are responsible for ensuring that inspection is
adequate. The assignment of this responsibility ensures that all product
requirements are met on a continuing basis.
6.0 RECORDS
6.1 Inspection records are documented on the incoming packing slip and on
the outgoing last copy of invoice. Gathering data will be performed and
maintained by Quality Rep.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 3
Title:
Monitoring and
Measurement
Effective date:
11/22/06
Revision number:
6.0
Section :
P
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the requirements for monitoring and measuring
the performance of the QMS.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Paragraph 8.2
2.2
Quality System Procedure – 8.2 Customer Satisfaction Survey
2.2
Quality System Procedure – 17.5 Internal Quality Audits
2.2
Quality System Procedure – 10.5 Receiving
2.2
Quality System Procedure – 10.6 Receiving Inspection (for future use)
2.2
Quality System Procedure – 10.7 Final Inspection
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Customer Satisfaction (8.2.1)
4.1.1 IBS measures internal and external customer satisfaction by
understanding expectations and collecting data to measure performances. The
company competes to provide the most reliable parts and services in the
industry as stated in the quality policy.
4.1.2 IBS establishes measures of external customer satisfaction through
customer surveys and daily customer contacts. Improvement opportunities are
identified and initiated by the responsible employees.
4.1.3 Internal customer satisfaction is addressed by implementing the
continual improvement model. This process establishes the customer
satisfaction philosophy, performance measures, information collection and
analysis of issues. The underlying principle for internal customer satisfaction is
that we are all on the same team and together we can make things happen in
a positive manner. ( Ref.: Section, D, Para 4.2, Customer Focus)
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 3
Title:
Monitoring and
Measurement
Effective date:
11/22/06
Revision number:
6.0
Section :
P
Applicability: This section is applicable to all
IBS Electronics
operations.
4.2
Internal Audit (8.2.2)
4.2.1 A system of planned and documented internal audits is established. The
purpose of the program is to ensure that the quality policy, procedures
accurately reflect the internal processes. Audit results are used to determine
the effectiveness of implementation of the quality program. A prescribed audit
checklist is utilized for each audit.
4.2.2 The results of audits are reviewed and are compared with previous
audits by the management. Corrective actions are implemented.
4.3
Monitoring and Measurement of Processes (8.2.3)
4.3.1 The suitable methods for monitoring quality system are defined by
management of IBS where applicable. Through identification of processes by
process flow chart, customer surveys, internal audits, sales performance,
nonconformance records and management reviews results.
4.4
Monitoring and Measurement of Product (8.2.4)
4.4.1 Inspection is performed in accordance with documented procedures.
Measurement of the quality of received part is through visual inspection.
4.4.2 Verification of part is performed in accordance with the requirements of
purchase order through documented procedures.
4.4.3 All material received is verified by receiving against the purchase order
(as a minimum) for correct supplier, marking, and quantity.
4.4.4 A visual inspection for damage to the packaging is performed before
being put into stock.
4.4.5 A final inspection is done on the part to ensure that it meets customer
requirements. All part receives a final visual inspection. The inspection is
performed in accordance with the requirements of sales order through
documented procedures.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 3
Title:
Monitoring and
Measurement
Effective date:
11/22/06
Revision number:
6.0
Section :
P
Applicability: This section is applicable to all
IBS Electronics
operations.
5.0 RESPONSIBILITIES
5.1 All employees are responsible for providing internal and external customer
satisfaction and implementing and communicating alternative actions when
necessary.
5.2 The Quality Rep. is responsible for the audit process to include selecting,
training auditor(s), managing, scheduling audits, generating audit reports and
tracking corrective actions for continual improvement.
5.3 Documented procedures are generated as required to establish part
conformance to the requirements of purchase order. Sales, receiving and
quality are responsible for ensuring that inspection is adequate. The
assignment of this responsibility ensures that all part requirements are met on
a continuing basis.
6.0 RECORDS
6.1 Customer surveys, sales performance report, customer feedback records
are maintained by sales group and quality representative.
6.2 Records of internal audits are maintained by the Quality Representatives.
Inspection records are documented on the incoming packing slip and on the
outgoing last copy of invoice.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 1
Title:
Control of Nonconforming
Product
Effective date:
11/22/06
Revision number:
6.0
Section :
Q
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0
PURPOSE
1.0 This section establishes the requirements for controlling nonconforming
product
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Paragraph 8.3
2.2
Quality System Procedure – 13.5 Control of Nonconforming Material
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0 QMS
REQUIREMENTS
4.1
Control of Nonconforming Product (8.3)
4.1.1 Nonconforming materials received by suppliers or customer return are
identified and segregated.
4.1.2 Nonconforming materials are not shipped. An evaluation is employed to
determine the disposition and action required to purge system of non
conforming materials.
4.1.3 Following an evaluation, discrepant materials may be dispositioned for
use as is (re-stock), scrap or return to supplier.
5.0 RESPONSIBILITIES
5.1 Nonconforming materials are reviewed by Quality Rep. or authorized buyer
to determined whether they should be rejected, scrapped or return to supplier.
6.0 RECORDS
6.1 The Quality Rep. and receiving maintain records of rejected parts
.
The
nature of nonconformities and any subsequent actions taken, are
documented and maintained.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 2
Title:
Analysis of Data
Effective date:
11/22/06
Revision number:
6.0
Section :
R
Applicability: This section is applicable to all
IBS Electronics
operations.
1.0 PURPOSE
1.1 This section establishes the requirements for the analysis of data regarding the
QMS.
2.0 REFERENCE DOCUMENTS
2.1
ISO 9001:2000 Paragraph 8.4
2.2
Quality System Procedure – 20.1
Sales & Shipping Performance
Measurement
2.3
Quality System Procedure – 6.8 Supplier Performance Measurement
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions
4.0
QMS REQUIREMENTS (8.4)
4.1
Analysis of Data (8.4)
4.1.1 The analyzing of data is defined by management of IBS as a tool for
determining suitability and monitoring the process; and identifying
improvements. Any procedures relating to analysis of data are to be found in
this section.
4.1.2 The determination of specific analysis of data requirements depend on
the need for control as defined by management.
h)
Customer complaints and customer satisfaction report,
i)
findings from internal quality system audits
j) nonconformance
records
k) supplier
performance
l)
statistical process analysis on sales performance
m)
results from management reviews
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 2
Title:
Analysis of Data
Effective date:
11/22/06
Revision number:
6.0
Section :
R
Applicability: This section is applicable to all
IBS Electronics
operations.
5.0 RESPONSIBILITIES
5.1 The Quality Rep. is responsible for implementing the method of and
procedures that will measure the process performance as required.
6.0 RECORDS
6.1 Gathering data will be performed and maintained by Quality Rep.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 3
Title:
Improvement
Effective date:
11/22/06
Revision number:
6.0
Section :
S
Applicability: This section is applicable to all IBS Electronics, Inc. operations.
1.0 PURPOSE
1.1 This section establishes the requirements for ensuring
continual improvement
of
the effectiveness of the quality management system.
2.0 REFERENCE
DOCUMENTS
2.1
ISO 9001:2000 Paragraph 8.5
2.3
Quality System Procedure – 14.5 Supplier Corrective Action
2.4
Quality System Procedure – 14.8 Preventive Action
2.5
Quality System Procedure – 14.6 Internal Corrective Action
2.6
Quality System Procedure – 14.7 Customer Complaints
3.0 DEFINITIONS
3.1
See Section T, Glossary, for definitions.
4.0 QMS
REQUIREMENTS
4.1
Continual Improvement (8.5.1)
4.1.1 A quality Continual improvement program is established to continually
improve the quality of parts and services. The process is to provide quality
parts and services in response to customer requirements that are subjected to
order reviews, customer feedback and RMA analysis.
4.1.2 IBS established and maintained a continuing training program for all new
employees.
4.1.3 The action projects are implemented and maintained within IBS as
continual improvement tools to identify and correct deficiencies in processes
and products
4.2
Corrective Action (8.5.2)
4.2.1 Corrective action is taken for all discrepancies based upon the degree of
the discrepancy found and its potential adverse effect on the quality system.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 3
Title:
Improvement
Effective date:
11/22/06
Revision number:
6.0
Section :
S
Applicability: This section is applicable to all IBS Electronics, Inc. operations.
4.2.2 The corrective action projects are based on the problem identified and
will be resolved through any of below data, as required.
customer satisfaction report,
analysis of non conforming materials,
findings from internal quality system audits and feedbacks,
supplier performance report,
analysis on sales performance report,
results from management reviews Internal corrective action process
records of customer complaints
ISO 9001-2000 Surveillance audit
4.2.3 IBS implements and records any changes to documented procedures
resulting from corrective action.
4.3
Preventive Action (8.5.3)
4.3.1 Preventive action is identification of sources of non-conformities results
in action being taken to correct processes and/or procedures as necessary to
prevent their occurrence.
4.3.2 Preventive action items are clearly defined, persons are assigned and
completion dates are established.
4.3.3 Preventive actions can be initiated and followed up by preventive
planning report created for management review meeting. The report should
define preventive plan assigned person and completion date.
4.3.4 Preventive actions may be performed through corrective action
procedure.
4.3.5 Preventive planning and actions are taken which are not covered in
procedures but which utilize continual improvement to implement process
changes and facilities changes before discrepancies or non conformities occur.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 3
Title:
Improvement
Effective date:
11/22/06
Revision number:
6.0
Section :
S
Applicability: This section is applicable to all IBS Electronics, Inc. operations.
4.3.6 These include ESD damage prevention, moving to new larger facility,
enhancements of computer hardware and software tools. These actions are
not documented but are evidence of preventive action planning effectiveness.
4.3.7 The Corrective and Preventive Action procedure is a combined
Quality System Procedure, which makes a clear distinction between
corrective action and preventive action and their inter-relationship.
4.3.8 IBS
implements and records any changes to documented procedures
resulting from preventive action(s).
5.0 RESPONSIBILITIES
5.1 The General Manager verbalizes the goals and objectives of quality
improvement to all employees. Customer satisfaction is principal, continual
improvement is essential upon all employees. IBS’ goals include appropriate
measures of quality improvement and customer satisfaction.
5.2 Requests for corrective actions may be made at any time by any member
of IBS team. The Quality Rep. will evaluate, follow up corrective actions.
6.0 RECORDS
6.1 The Quality rep. maintains records of continual improvement, corrective
and preventive actions. Results of improvement, corrective and preventive
actions are discussed at the Management Review meetings.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
TERMS AND DEFINITIONS
AUDIT
– Systematic, independent, and documented
process
for obtaining
audit evidence
and
evaluating it objectively to determine the extent to which
audit criteria
are fulfilled [ISO 9000:2000 -
3.9.1].
AUDIT CRITERIA
– Set of policies,
procedures,
or
requirements
used as a reference [ISO
9000:2000 - 3.9.3].
AUDIT CONCLUSION
– Outcome of an
audit
provided by the
audit team
after consideration of the
audit objectives and all
audit findings
[ISO 9000:2000 - 3.9.6].
AUDIT EVIDENCE - Records
, statements of fact, or other
information
which are relevant to the
audit criteria
and verifiable [ISO 9000:2000 - 3.9.4]
AUDIT FINDING
– Results of the evaluation of the collected
audit evidence
against
audit criteria
.
(Note: Audit findings can indicate either conformity or nonconformity with audit criteria, or
opportunities for improvement. [ISO 9000:2000 3.9.5]).
AUDIT PROGRAM –
Set of one or more
audit
s planned for a specific time frame and directed
towards a specific purpose. (Note: One auditor in the audit team is generally appointed as audit team
leader) [ISO 9000:2000 - 3.9.2].
AUDIT TEAM –
One or more
auditors
conducting an
audit
[ISO 9000:2000 - 3.9.10].
AUDITEE –
Organization
being audited [ISO 9000:2000 - 3.9.8].
AUDITOR
– Person with the
competence
to conduct an
audit
[ISO 9000:2000 - 3.9.9].
CAPABILITY -
Ability of an
organization
,
system
, or
process
to realize a
product
that will fulfill the
requirements
for that
product
[ISO 9000:2000 - 3.1.5]
CHARACTERISTIC
– Distinguishing feature [ISO 9000:2000 - 3.5.1].
COMPETENCE –
Demonstrated ability to apply knowledge and skills [ISO 9000:2000 – 3.9.12].
CONCESSION
– Permission to use or release a
product
that does not conform to specified
requirements
[ISO 9000:2000 - 3.6.11].
CONFORMITY –
Fulfillment of a
requirement
[ISO 9000:2000 - 3.6.1].
CONTINUAL IMPROVEMENT
– A recurring activity to increase the ability to fulfill
requirements
[ISO 9000:2000 - 3.2.13]
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
2 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
CORRECTION
– Action taken to eliminate a detected
nonconformity
[ISO 9000:2000 - 3.6.6].
CORRECTIVE ACTION -
Action to eliminate the cause of a detected
nonconformity
or other
undesirable situation (Note: There is a distinction between
correction
and
corrective action
)[ISO
9000:2000 - 3.6.5].
CUSTOMER
–
Organization
or person that receives a
product
(or service) [ISO 9000:2000 - 3.3.5].
CUSTOMER SATISFACTION
– Customer’s perception of the degree to which the customer’s
requirements
have been fulfilled [ISO 9000:2000 - 3.1.4].
DEFECT -
Non-fulfillment of a
requirement
related to an intended or specified use (NOTE: The
distinction between defect and nonconformity is important as it has legal connotations, particularly
those associated with product liability issues; consequently, the term “defect” should be used with
extreme caution)[ISO 9000:2000 - 3.6.3].
DESIGN AND DEVELOPMENT
– Set of
processes
that transforms
requirements
into specified
characteristics
or into the
specification
of a
product
,
process,
or
system
[ISO 9000:2000 - 3.4.4].
DEVIATION PERMIT-
Permission to depart from the originally specified
requirements
of a
product
prior to realization [ISO 9000:2000 - 3.6.12].
DOCUMENT
–
Information
and its supporting medium [ISO 9000:2000 - 3.7.2].
EFFECTIVENESS -
Extent to which planned activities are realized and planned results are achieved
[ISO 9000:2000 - 3.2.14].
FOLLOW-UP AUDIT -
A special audit performed to verify that corrective action has been
implemented as scheduled and that the action was effective in preventing or minimizing recurrence.
INDEPENDENCE -
Freedom from bias and external influence; provides for objectivity and
impartiality.
INFORMATION -
Meaningful data [ISO 9000:2000 - 3.7.1].
INFRASTRUCTURE -
System of facilities, equipment, and services needed for the operation of an
organization
[ISO 9000:2000 - 3.3.3].
INSPECTION –
Conformity evaluation by observation and judgement—accompanied, as
appropriate, by measurement, testing, or gauging [ISO 9000:2000 - 3.8.2].
INSPECTION RECORD -
Document stating results (data) concerning inspection activities.
LEAD AUDITOR -
The individual who manages the
audit team
during an
audit
.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
3 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
MANAGEMENT SYSTEM
– A
system
to establish policy and objectives and to achieve those
objectives [ISO 9000:2000 - 3.2.2].
MEASUREMENT CONTROL SYSTEM
– Set of interrelated or interacting elements necessary to
achieve
metrological confirmation
and continual control of measurement processes [ISO 9000:2000 -
3.10.1].
MEASUREMENT PROCESS
– Set of operations to determine the value of a quantity [ISO
9000:2000 - 3.10.2].
METROLOGICAL CONFIRMATION
– Set of operations required to ensure that
measuring
equipment
conforms to the
requirements
for its intended use. (Note: Generally includes calibration or
verification, any necessary adjustment or repair, and subsequent recalibration, comparison with the
metrological requirements for the intended use of the equipment, as well as any required sealing and
labeling) [ISO 9000:2000 - 3.10.3].
MEASURING EQUIPMENT
– Measuring instrument, software, measurement standard, reference
material, or auxiliary apparatus or combination thereof necessary to realize a
measurement process
[ISO 9000:2000 - 3.10.4].
METROLOGICAL CHARACTERISTIC
– Distinguishing feature which can influence the results
of measurement [ISO 9000:2000 - 3.10.5].
METROLOGICAL FUNCTION
- Function with organizational responsibility for defining and
implementing the
measurement control system
[ISO 9000:2000 - 3.10.6].
NONCONFORMITY –
Non-fulfillment of a
requirement
[ISO 9000:2000 3.6.2].
OBJECTIVE EVIDENCE –
Data supporting the existence or verity of something [ISO 9000:2000 -
3.8.1]
OBSERVATION –
A concern or weakness detected in an element in the management system, but not
a nonconformance; a condition that may become a nonconformance if not addressed; an opportunity
for improvement.
OPENING MEETING –
The introductory meeting between the auditor(s) and the auditee’s
representative, during which the overview of the planned audit is presented.
ORGANIZATION
– Group of people and facilities with an arrangement of responsibilities,
authorities, and relationships [ISO 9000:2000 - 3.3.1].
ORGANIZATIONAL STRUCTURE
– Arrangement of responsibilities, authorities, and
relationships between people [ISO 9000:2000 - 3.3.2].
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
4 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
PRE-AWARD SURVEY -
An activity conducted prior to a contract award and used to evaluate the
overall quality capability of a prospective supplier or contractor.
PREVENTIVE ACTION
– Action to eliminate the cause of a potential
nonconformity
or other
undesirable potential situation (Note: Preventive action is taken to prevent occurrence, whereas
corrective action is taken to prevent recurrence) [ISO 9000:2000 - 3.6.4].
PROCEDURE -
Specified way to carry out an activity or
process
[ISO 9000:2000 - 3.4.5].
PROCESS –
Set of interrelated or interacting activities which transforms inputs into outputs. (Note 1:
Inputs to a process are generally outputs from other processes. Note 2: Processes in an organization
are generally planned and carried out under controlled conditions to add value. Note 3: A process
where the conformity of the resulting product cannot be readily or economically verified is frequently
referred to as a “special process”) [ISO 9000:2000 - 3.4.1].
PRODUCT –
Result of a
process
. (Note 1: There are four generic categories of product: 1)
Services
,
2) Software, 3) Hardware, 4) Processed materials [ISO 9000:2000 - 3.4.2].
Note: For the purposes of its ISO 9000-2000 Certification,
IBS Electronics
’s product consists
of the
PROJECT
– Unique
process
, consisting of a set of coordinated and controlled activities with start
and finish dates, undertaken to achieve an objective conforming to specific
requirements
, including
the constraints of time, cost, and resources [ISO 9000:2000 - 3.4.3].
QUALITY
–
Degree to which a set of inherent
characteristics
fulfills
requirements
[ISO 9000:2000 -
3.1.1].
QUALITY ASSURANCE –
Part of
quality management
focused on providing confidence that
quality
requirements
will be fulfilled [ISO 9000:2000 - 3.2.11].
QUALITY CONTROL –
Part of
quality management
focused on fulfilling quality
requirements
[ISO 9000:2000 - 3.2.10].
QUALITY IMPROVEMENT
–
Part of
quality management
focused on increasing the ability to
fulfill quality
requirements
[ISO 9000:2000 - 3.2.12].
QUALITY MANAGEMENT SYSTEM (QMS)
– A
management system
to direct and control an
organization
with regard to
quality
[ISO 9000:2000 - 3.2.3].
QUALITY MANUAL (QM) -
Document
specifying the
quality management system
of an
organization
[ISO 9000:2000 - 3.7.4].
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
5 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
QUALITY OBJECTIVE -
Something sought, or aimed for, related to quality (Note 1: Quality
objectives are generally based on the organization’s
quality policy
; Note 2: Quality objectives are
generally specified for relevant functions and levels in the organization)[ISO 9000:2000 - 3.1.1].
QUALITY PLAN -
Document
specifying which
procedures
and associated
resources
shall be
applied by whom and when to a specific
project
,
product
,
process,
or contract [ISO 9000:2000 -
3.7.5].
QUALITY PLANNING –
Part of
quality management
focused on setting
quality objectives
and
specifying necessary operational
processes
and related
resources
to fulfill the
quality objectives
[ISO
9000:2000 - 3.2.9].
QUALITY POLICY -
The overall intentions and direction of an
organization
related to
quality
as
formally expressed by
top management
[ISO 9000:2000 - 3.2.4].
RECORD -
Document
stating results achieved or providing evidence of activities performed [ISO
9000:2000 - 3.7.6].
RELEASE -
Permission to proceed to the next stage of a process [ISO 9000:2000 - 3.6.13].
REQUIREMENT -
Need or expectation that is stated, generally implied, or obligatory [ISO
9000:2000 - 3.1.2].
RESOURCES -
People, time, money, buildings, equipment, and support activities, as necessary, that
may be applied to a specific project, product, process, and/or contract in order to fulfill
requirements
.
REVIEW
– Activity undertaken to determine the suitability, adequacy, and
effectiveness
of the
subject matter to achieve established objectives [ISO 9000:2000 - 3.8.7].
ROOT CAUSE -
The fundamental deficiency that results in a nonconformance that must be
eliminated through corrective action to prevent recurrence of the same or similar nonconformance.
ROOT CAUSE ANALYSIS -
Investigation to determine the fundamental deficiency that resulted in
a nonconformity.
SERVICE
– The result of at least one activity necessarily performed at the interface between the
supplier and the customer and that is generally intangible. Provision of a service can involve: 1)
Activity performed on a customer-supplied tangible product, 2) Activity performed on a customer-
supplied intangible product, 3) Delivery of an intangible product, 4) Creation of ambience for the
customer [ISO 9000:2000 - 3.4.2 Note 2].
SPECIFICATION –
Document
stating
requirements
[ISO 9000:2000 - 3.7.3].
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
6 of 6
Title:
Glossary (terms and
Definition)
Effective date:
11/22/06
Revision number:
6.0
Section :
T
Applicability: This section is applicable to all
IBS Electronics
operations.
SUPPLIER
–
Organization
or person that provides a
product
[ISO 9000:2000 - 3.3.6].
SYSTEM -
Set of interrelated or interacting elements [ISO 9000:2000 - 3.2.1]
TEST –
Determination of one or more
characteristics
according to a
procedure
[ISO 9000:2000 -
3.8.3].
TOP MANAGEMENT
– Person or group of people who directs and controls an
organization
at the
highest level [ISO 9000:2000 - 3.2.7].
TRACEABILITY -
Ability to trace the history, application, or location of that which is under
consideration [ISO 9000:2000 - 3.5.4].
VALIDATION
– Confirmation, through the provision of
objective evidence
, that the
requirements
for
a specific intended use or application have been fulfilled [ISO 9000:2000 - 3.8.5].
VERIFICATION –
Confirmation, through the provision of
objective evidence
, that specified
requirements
have been fulfilled [ISO 9000:2000 - 3.8.4].
WORK ENVIRONMENT
- Set of conditions under which work is performed (Note: Conditions
include physical, social, psychological and environmental factors (temperature, recognition schemes,
ergonomics and atmospheric composition)) [ISO 9000:2000 - 3.3.4]
.
quality_policy_manual_v6-html.html
IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL
Prepared by:
Shawn Mouzoon
Approved by:
Shawn Mouzoon
Page:
1 of 1
Title:
Record of Revision
Effective date:
11/22/06
Revision number:
6.0
Section :
U
Applicability: This section is applicable to all
IBS Electronics
operations.
Revision Section
Detail
Effective
Date
1.0
All
Initial Issue of the 9001:2000 transition revision to the
QSM
08/01/03
2.0
B,G,H,J Section B, Para 1.0 : removed calibration exclusion of
element 7.6.
Section B, para 4.2 & 4.5: revised 4.2.1, added 4.2.2, 4.2.3,
4.2.4, 4.5.2 (4.1) (5.4.1).
Section G, para 4.2: revised 4.2.3.6 .
Section H, para 4.3: revised 4.3.4, added 4.3.5 (6.2.2).
Section J, para 4.1 and 4.2: revised 4.1.2 and 4.2.2.(7.2.1).
.
10/10/03
3.0
H
Section B, Para 4.2.2; Section H, Master Operation Flow
Chart
06/15/05
4.0.
D, J, P.
Section D, Para 4.2, revised 4.2.1
Section J, Para 4.1, revised 4.1.2
Section P, Para, 4.1, revised 4.1.3
01/09/06
5.0.
S.
Section S, Para 4.2, revised 4.2.2
05/26/06
6.0
B, F, M,
N
Section B, Para 1.0, revised 1.1
Section B, Para 4.2, revised 4.2.2
Section F, Para 4.2, revised 4.2.1
Section F, Para 4.3, revised 4.3.1
Section M, Para 4.2, revised 4.2.1, deleted 4.2.2 & 4.2.3
Section N, Para, 1.0, revised 1.2
11/22/06
.
quality_policy_manual_v6-html.html

IBS Electronics, Inc
.
QUALITY SYSTEM MANUAL


























































